When a single twin plane is formed, the resulting nucleus, at the early stage of growth, is a hexagonal plate due to the 6-fold symmetry of fcc structures (Figure 13). Each side of the hexagon is either a concave side or a convex side because of the presence of a twin plane. When atoms diffuse toward the nucleus and adsorb to grow the NC, they can adsorb either on a concave or on a convex face. On the convex side, each atomic site has only three closest neighbors, and the stabilization energy is therefore quite low. On the other hand, on the concave side, newly adsorbed atoms are stabilized by four neighbors from several facets, increasing the stabilization energy. Atoms will then preferentially adsorb on the concave sides, favoring the growth of the NC along these sides rather than the convex sides. The growth will be favored on the facets that present a concave-type surface, while the convex-type surface does not favor adsorption of new atoms and will remain unchanged. This can also be explained by the Gibbs−Thomson formula which states that the chemical potential is inversely proportional to the curvature. Δμ γ = Ω + (1/ R1+1/ R2)
where Δμ is the change in the chemical potential, γ the interfacial free energy, Ω the atomic volume, and R1 and R2 are the radii curvature.2 For a convex surface where the curvature is positive, the chemical potential change is positive and the adsorption of atoms is favored on a flat surface rather that a convex surface. On the other hand, for a concave surface where the curvature is negative, the change in the chemical is negative and the adsorption of atoms on the concave side is favored.
TIM截图20200715203155.jpg
When a single twin plane is formed, the resulting nucleus, at the early stage of growth, is a hexagonal plate due to the 6-fold symmetry of fcc structures (Figure 13). Each side of the hexagon is either a concave side or a convex side because of the presence of a twin plane. When atoms diffuse toward the nucleus and adsorb to grow the NC, they can adsorb either on a concave or on a convex face. On the convex side, each atomic site has only three closest neighbors, and the stabilization energy is therefore quite low. On the other hand, on the concave side, newly adsorbed atoms are stabilized by four neighbors from several facets, increasing the stabilization energy. Atoms will then preferentially adsorb on the concave sides, favoring the growth of the NC along these sides rather than the convex sides. The growth will be favored on the facets that present a concave-type surface, while the convex-type surface does not favor adsorption of new atoms and will remain unchanged. This can also be explained by the Gibbs−Thomson formula which states that the chemical potential is inversely proportional to the curvature. Δμ γ = Ω + (1/ R1+1/ R2)
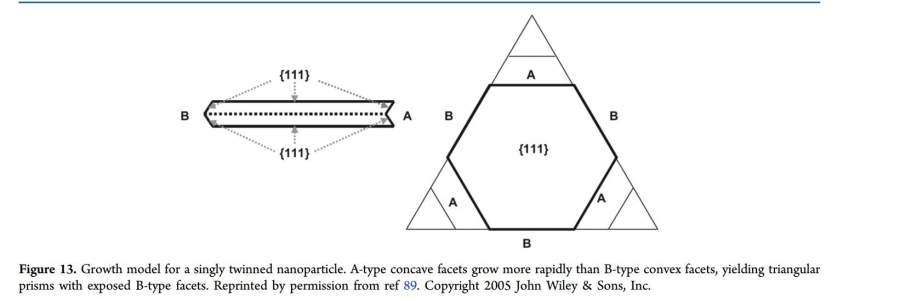
where Δμ is the change in the chemical potential, γ the interfacial free energy, Ω the atomic volume, and R1 and R2 are the radii curvature.2 For a convex surface where the curvature is positive, the chemical potential change is positive and the adsorption of atoms is favored on a flat surface rather that a convex surface. On the other hand, for a concave surface where the curvature is negative, the change in the chemical is negative and the adsorption of atoms on the concave side is favored.
TIM截图20200715203155.jpg
TIM截图20200715204028.jpg
,