吡啶4号位甲基溴代反应问题
请教一下,我在做吡啶衍生物4号位上的甲基溴代反应(见图),图片顺序可能有问题,请见谅
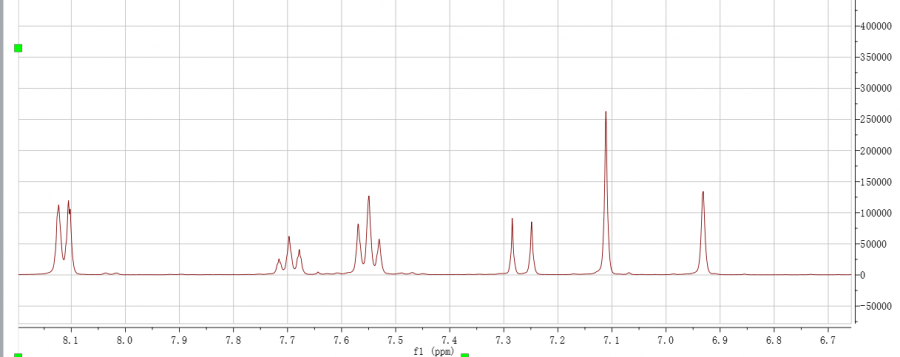
1. 核磁打谱在8左右附近有清晰的二重三重峰(见图),不应该啊,请问是为什么?图是第一次的谱,4左右没出峰,没产物
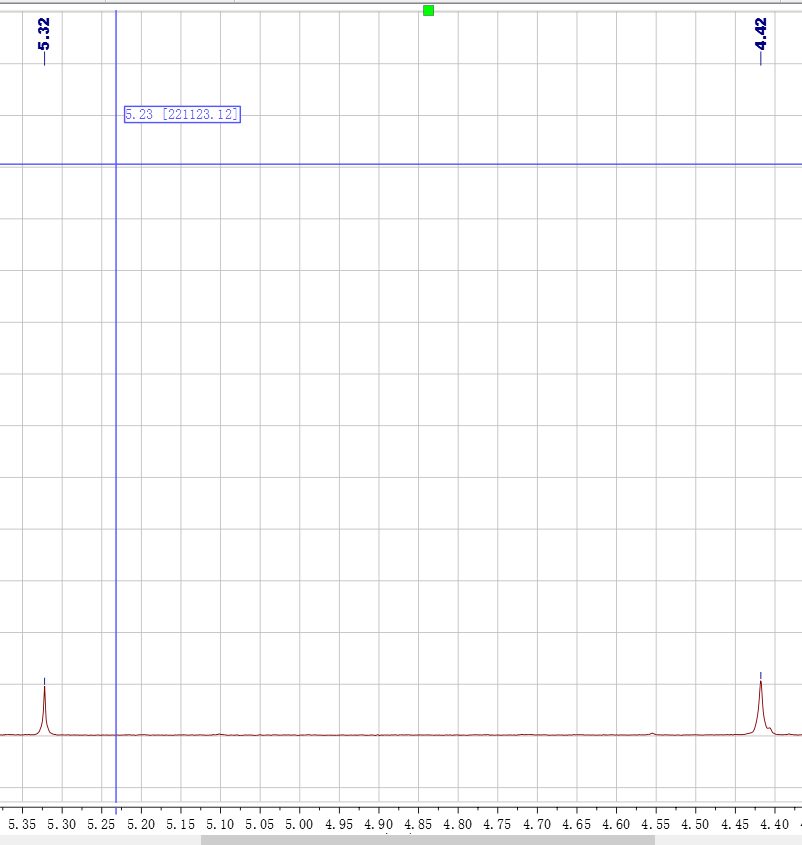
2. 后来用鼓泡法(见图),最后4ppm 产物峰出来了,但是5左右还有峰,是二溴代了吗?而且8左右还是有二、三重峰
3. 溶剂四氯化碳用分子筛除水,1.2当量NBS分三次加,65℃回流4h,BPO 0.12当量,产率比较低,请问如何改进?
4. 过柱子纯石油醚不加压,原料点和产物点也不好分,虽然跑板分得很开,是因为硅胶酸性吗?

IMG_20200709_084709.jpg
KL~%I)TH`REKHSI{QHY0)GD.png
VDWF`K})9AIXE2[[UJO)DA2.png
G2DPIV[N(JVE_[%~XJBFV58.png
P5I~23PLX%0221ESM3_Z6TE.png@liuchong630@liuchong630@yangtie2008![]() 返回小木虫查看更多
返回小木虫查看更多
今日热帖



 京公网安备 11010802022153号
京公网安备 11010802022153号
有没有大佬
大佬们帮帮忙
首先你的原料也测试核磁了吗?这个反应容易出现2溴代物 TLC分点开的话 柱层析应该也能分开
2溴代55%收率
Benzylic brominations with N-bromosuccinimide in (trifluoromethyl)benzene
By Suarez, Diana et al
From Synthesis, (11), 1807-1810; 2009
1溴代
Reactions of 2,6-di-tert-butylpyridine derivatives with methyl fluorosulfonate under high pressure
By Hou, C. J. and Okamoto, Yoshiyuki
From Journal of Organic Chemistry, 47(10), 1977-9; 1982
Benzylic brominations with N-bromosuccinimide in (trifluoromethyl)benzene
By Suarez, Diana et al
From Synthesis, (11), 1807-1810; 2009
Synthesis of Some Fluorinated Pyridines Using Tetrabutylammonium Fluoride
By Mobinikhaledi, A. and Foroughifar, N.
From Phosphorus, Sulfur and Silicon and the Related Elements, 181(2), 405-412; 2006
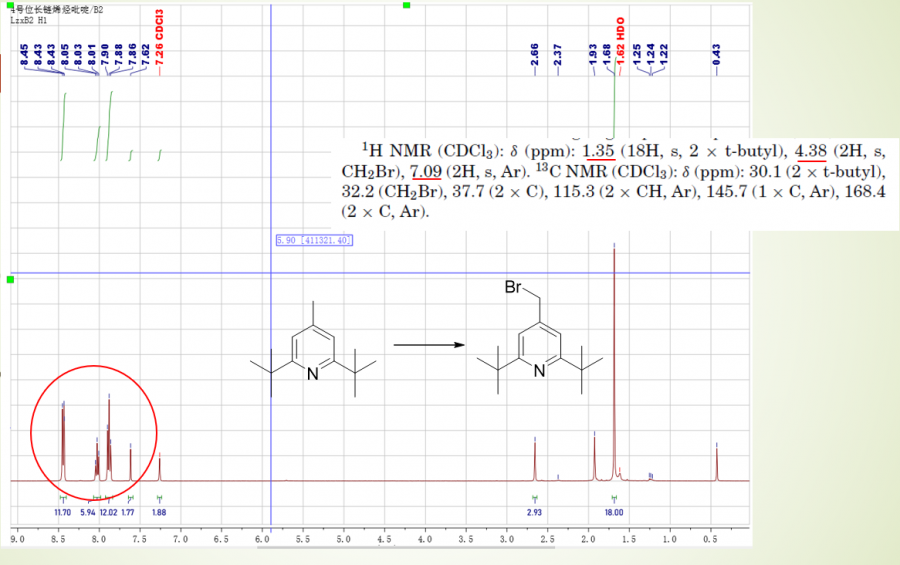
4-(Bromomethyl)-2,6-di-tert-butylpyridine (4a) A mixture of 2,6-di-tert-butyl-4-methylpyridine, 1, (0.21 g, 1.0 mmol), NBS (0.18 g, 1 mmol), and dibenzoyl peroxide (0.025 g, 1 mmol) in dry carbon tetrachloride (15 mL) was refluxed under N2 for 3 h. The mixture was then cooled in an ice bath and the precipitated succinimide was removed by filtration. The solvent was evaporated to give a brown oily mixture. This mixture was dissolved in carbon tetrachloride (15 mL). NBS (0.036 g, 0.20 mmol) and dibenzoyl peroxide (0.0050 g, 0.021 mmol) added and refluxed under N2 for another 2 h. The crude product was distilled at 90-110°C/2 mm Hg to give pure compound 4a (93%). 4-(Bromomethyl)-2,6-di-tert-butylpyridine (4a), yield 93%. 1H NMR (CDCl3): δ (ppm): 1.35 (18H, s, 2 x t-butyl), 4.38 (2H, s, CH2Br), 7.09 (2H, s, Ar). 13C NMR (CDCl3): δ (ppm): 30.1 (2 x t-butyl), 32.2 (CH2Br), 37.7 (2 x C), 115.3 (2 x CH, Ar), 145.7 (1 x C, Ar), 168.4 (2 x C, Ar),
谢谢,这几篇我之前看了,还是用AIBN比较好
测了,但是我发现跑出来的两个点,原料和产物是在一起的。。。另一个就是那个芳基峰,我改用了AIBN,跑出来就一个点了,可能是BPO的问题