����H�ģ������仰ʲô��˼����л��
�����л�������ṹʽ���£���Ͷ��ƪ���¡�����˸��ظ��˹����������ʣ�����������˵Ļ���ʲô��˼����Ұ��ҷ����¡���л��������˵�����������������Ϊ�����壬δ����֣���
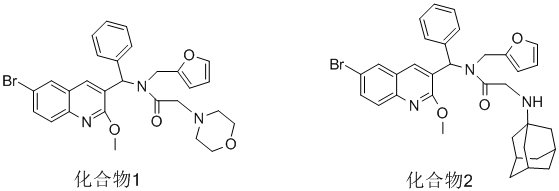
Compound 1: Only one chemical shift for each proton in the COCH2-beta and NCH2 AB systems must be calculated. The first
chemical shift for the NCH2 AB system is not correct because calculation implies a coupling constant of 165 Hz.
Compound 1: Only one chemical shift for each proton in the NCH2 AB system must be calculated.![]() ����Сľ��鿴����
����Сľ��鿴����
��������



 ���������� 11010802022153��
���������� 11010802022153��
���������д�ģ�������1�����ݣ�
1H NMR ( 500 MHz, CDCl3) ��: 2.47~2.57(m, 4H, 2��NCH2); 3.27~3.29(d, 1H, J=13.5Hz, COCH2-��); 3.41~3.44(d, 1H, J=13.5Hz, COCH2-��); 3.44~3.47(m, 4H, 2��OCH2); 4.10~4.43(d, J=15.5Hz, 1H, NCH2); 4.93~4.96(d, J=15.5Hz, 1H, NCH2); 5.75(s, 1H, furan-H20); 6.02(s, 1H, furan-H21); 7.68(s, 1H, furan-H22); 3.89(s, 3H, OCH3); 7.05(s, 1H, ArCH); 7.13~7.14(d, 2H, C6H5-H2,H6); 7.26~7.28(m, 1H, C6H5-H4); 7.35~7.37(m, 2H, C6H5-H3,H5); 7.37(s, 1H, quinoline ring-H7); 7.39(m, 1H, quinoline ring-H2); 7.65(m, 1H, quinoline ring-H3); 7.73(m, 1H, quinoline ring-H6).
������2�����ݣ�
1H NMR ( 500MHz, CDCl3) ��:1.51~2.09(m, 15H, adamantyl group); 3.46(d, 1H, J=13.5Hz,COCH2-��); 3.74(d, 1H, J=13.5Hz, COCH2-��); 3.96(s, 3H, OCH3); 4.08~4.11(d, J=15.5Hz, 1H, NCH2); 4.99~5.02(d, J=15.5Hz, 1H, NCH2); 5.70(s, 1H, furan-H20); 6.03(s, 1H, furan-H19); 7.68(s, 1H, furan-H18); 7.09(s, 1H, ArCH); 7.15~7.16(d, 2H, C6H5-H2, H6); 7.30~7.32(m, 1H, C6H5-H4); 7.39~7.41(m, 2H, C6H5-H3, H5); 7.39(s, 1H, quinoline ring-H7); 7.41(m, 1H, quinoline ring-H2); 7.65(m, 1H, quinoline ring-H3); 7.73(m, 1H, quinoline ring-H6)��
����4.10~4.43(d, J=15.5Hz, 1H, NCH2), ò����ϳ���Ӧ���ǣ�4.43-4.10��*500=165Hz�Dz��ǻ�ѧλ��ֵ����ˣ�������2 ��4.08~4.11(d, J=15.5Hz, 1H, NCH2); 4.99~5.02(d, J=15.5Hz, 1H, NCH2);�ǶԵģ�����ԱȽ�һ�¡�
������1 ��3.27~3.29(d, 1H, J=13.5Hz, COCH2-��); 3.41~3.44(d, 1H, J=13.5Hz, COCH2-��);�ͻ�����2��3.46(d, 1H, J=13.5Hz,COCH2-��); 3.74(d, 1H, J=13.5Hz, COCH2-��);ò�Ʊ�����һ�¡����˾�����ý�3.27~3.29(d, 1H, J=13.5Hz, COCH2-��); 3.41~3.44(d, 1H, J=13.5Hz, COCH2-��);��Ϊ3.28(d, 1H, J=13.5Hz, COCH2-��); 3.43(d, 1H, J=13.5Hz, COCH2-��); ����������һ�µĵط�Ҳ��Ҫ������
���˾���d������ǻ�ѧλ��д�ɶ������ƽ��ֵ��á�
4.10~4.43(d, J=15.5Hz, 1H, NCH2); 4.93~4.96(d, J=15.5Hz, 1H, NCH2);
��������������У�ABϵͳ�ı����Dz����ʵģ�
��������˵���˼��AB��ϵ��Ӧ���ǿ��Լ������ѧλ��ֵ������ֻ��һ����ѧλ��ֵ����ʵ�ϣ�֪��AB��ϵ��ʲô���ӵĻ����������⡣
¥�������ģ� 3.27~3.29(d, 1H, J=13.5Hz, COCH2-��); 3.41~3.44(d, 1H, J=13.5Hz, COCH2-��); 3.44~3.47(m, 4H, 2��OCH2); 4.10~4.43(d, J=15.5Hz, 1H, NCH2); 4.93~4.96(d, J=15.5Hz, 1H, NCH2); ��ѧλ��ֵȫ����һ����Χ��������һ��ȷ����λ��ֵ��
�����б�Ҫ��¥������ͼ���֣�������ͼ����ʾ���Dz���AB��ϵ������ǣ�¥����д��ֵ�����¿��ǣ�������ǣ�Ҳ�ÿ�����ʲô���������������˵�����
�ܷ��پ������ô�����أ��鷳����
�ԣ�ͬ��¥�ϡ�beta-HӦ��д��һ����ѧλ�ƣ�����ƽ��ֵ����Ӧ����һ����Χ���������alfa-H �� beta-H�IJ�ֵ��һ����ż�ϳ�����ô�����һ���ģ������Ǹ�����˵���NH2��ż�ϳ�������ˣ�λ�Ʋ�ֵ0.33��̫���˰ɡ������㿴�����ż�ϳ�������ĺ��������Ⱑ��4.93~4.96(d, J=15.5Hz, 1H, NCH2)��ѧλ�Ʋ�ֵ0.03��ż�ϳ�����ô������15.5�İ���
�ڶ���˵�Ļ���N-CH2Ӧ��д��һ������������һ����Χ��