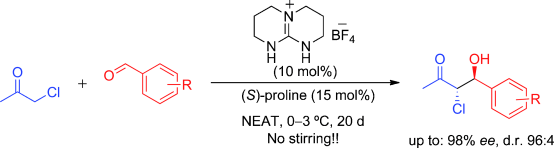
Proline-Catalysed Direct Aldol Reaction of Chloroacetone and Aromatic Aldehydes
The combination of proline and an achiral triazabicyclodecene-derived guanidinium salt permits, for the first time, the direct aldol reaction of chloroacetone and aromatic aldehydes (see scheme). The resulting chlorohydrins are formed with high regio-, diastereo- and enantioselectivity. This procedure is experimentally simple and green, working without solvent, in test tubes placed inside a standard laboratory fridge without agitation or mechanical stirring.
[ ���Կ��м��� ľ���л� ]
��������



 ���������� 11010802022153��
���������� 11010802022153��
���Σ�����ŷ��ѧ����д��CEJ��EJOC��ŷ���л���ѧ��һ����һ��ѧ�ƣ�һ���Ƕ���ѧ�ư��
���Գƺϳɡ��ֵ�Ҳ����ƪEJOC������Ҳ������showһ�¡�