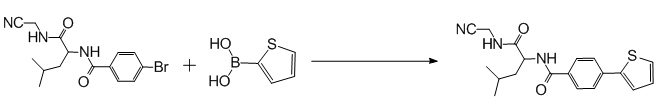
Suzuki反应 噻吩硼酸的偶合反应反应产率较低
本人用Suzuki反应,原料为2-噻吩硼酸和溴苯化合物,反应条件严格控制(http://muchong.com/bbs/viewthread.php?tid=7584645),但是产率一直不高,总有原料-溴苯化合物的存在。
网上看到有些推荐用硼酸酯反应,说硼酸酯的活性要高些;也有说对于噻吩类的物质一般要用stille反应而不用suzuki反应,做成Sn盐,活性较高。我权衡了一下,一是硼酸酯价格很贵,二是自己还从来没有过stille反应,两个方案都不敢贸然尝试,因此不知如何是好。
不知大家有没有碰到相同的反应,请大家给以建议。
QQ截图20140702155337.png![]() 返回小木虫查看更多
返回小木虫查看更多
今日热帖



 京公网安备 11010802022153号
京公网安备 11010802022153号
建议换过来做,苯环上做成硼酯和2-溴噻唑做Suzuki会好很多。
另外试试Pd(ph3p)4/K2CO3/DMF-H2O (8:1), 100度,微波30分钟
Agree. Phenylboronic acid is pretty cheap and 2-bromothiophene is very easy to make. Boronic acid is actually more active than ester. Probably can add more eq of Phenylboronic acid which can be easily separated afterward.
Pd(PPh3)2Cl2 can be used as catalyst, too, since sometimes the Pd(PPh3)4 might already lose its reactivity. However, it is important to make sure that there is no oxygen in the system, i.e., using a good Schlenk line or freeze-pump-thaw several cycles to remove the residue air.
这个苯环结构做硼酸酯不好做吧
苯环上做成硼酸酯对我来说很困难。如果我用噻吩硼酸酯反应的话,产率会不会有提高?方向杂环硼酸Suzuki反应是不是比非杂环芳香硼酸要难啊?
是的,不好做的 基本没可能
Boronic acid is actually more active than ester?But most people hold the opinion that ester is more active than boronic acid,do you have some evidences to support your opinion?
http://anderson.chem.ox.ac.uk/fi ... 14-suzuki-boron.pdf
See above link. You need some base to activate boron first, so boronic acid should be more reactive than boronic ester. Sometimes people have trouble because some boronic acid compounds are not stable.
Sorry I didn't see your structures this morning. But if you still recover phenylbromide as the starting material, maybe you can add more catalyst and run for a longer time.
BTW, can you make a Grignard reagent from 2-bromothiophene, then react with trimethylborate to generate your thiophenyl boronic ester or acid? Also, you might use freeze-pump-thaw trick to remove the air residue
,